Inhibitory control of correlated intrinsic variability in cortical networks
Cortical networks exhibit large-scale fluctuations which creates noise correlations that impact sensory coding. Modeling and data analysis show that inhibition can control these fluctuations.
Abstract
Cortical networks exhibit intrinsic dynamics that drive coordinated, large-scale fluctuations across neuronal populations and create noise correlations that impact sensory coding. To investigate the network-level mechanisms that underlie these dynamics, we developed novel computational techniques to fit a deterministic spiking network model directly to multi-neuron recordings from different rodent species, sensory modalities, and behavioral states. The model generated correlated variability without external noise and accurately reproduced the diverse activity patterns in our recordings. Analysis of the model parameters suggested that differences in noise correlations across recordings were due primarily to differences in the strength of feedback inhibition. Further analysis of our recordings confirmed that putative inhibitory neurons were indeed more active during desynchronized cortical states with weak noise correlations. Our results demonstrate that network models with intrinsically-generated variability can accurately reproduce the activity patterns observed in multi-neuron recordings and suggest that inhibition modulates the interactions between intrinsic dynamics and sensory inputs to control the strength of noise correlations.
Our brains contain billions of neurons, which are continually producing electrical signals to relay information around the brain. Yet most of our knowledge of how the brain works comes from studying the activity of one neuron at a time. Recently, studies of multiple neurons have shown that they tend to be active together in short bursts called “up” states, which are followed by periods in which they are less active called “down” states. When we are sleeping or under a general anesthetic, the neurons may be completely silent during down states, but when we are awake the difference in activity between the two states is usually less extreme. However, it is still not clear how the neurons generate these patterns of activity.
To address this question, we studied the activity of neurons in the brains of awake and anesthetized rats, mice and gerbils. The experiments recorded electrical activity from many neurons at the same time and found a wide range of different activity patterns:

We built a spiking neuronal network model which recapitulated the up and down states in the data:
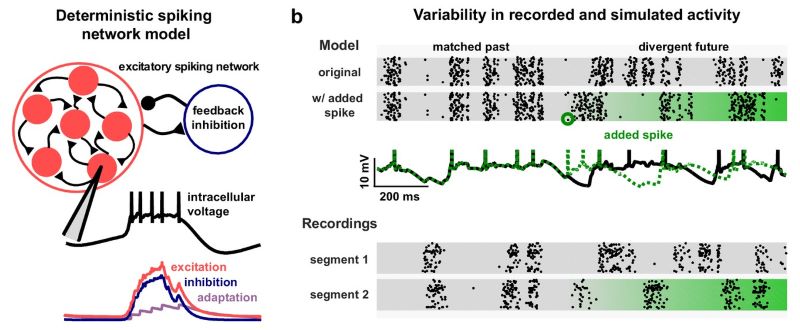
We fit the model parameters directly to the neural activity, and reproduced the spiking patterns across brain states:
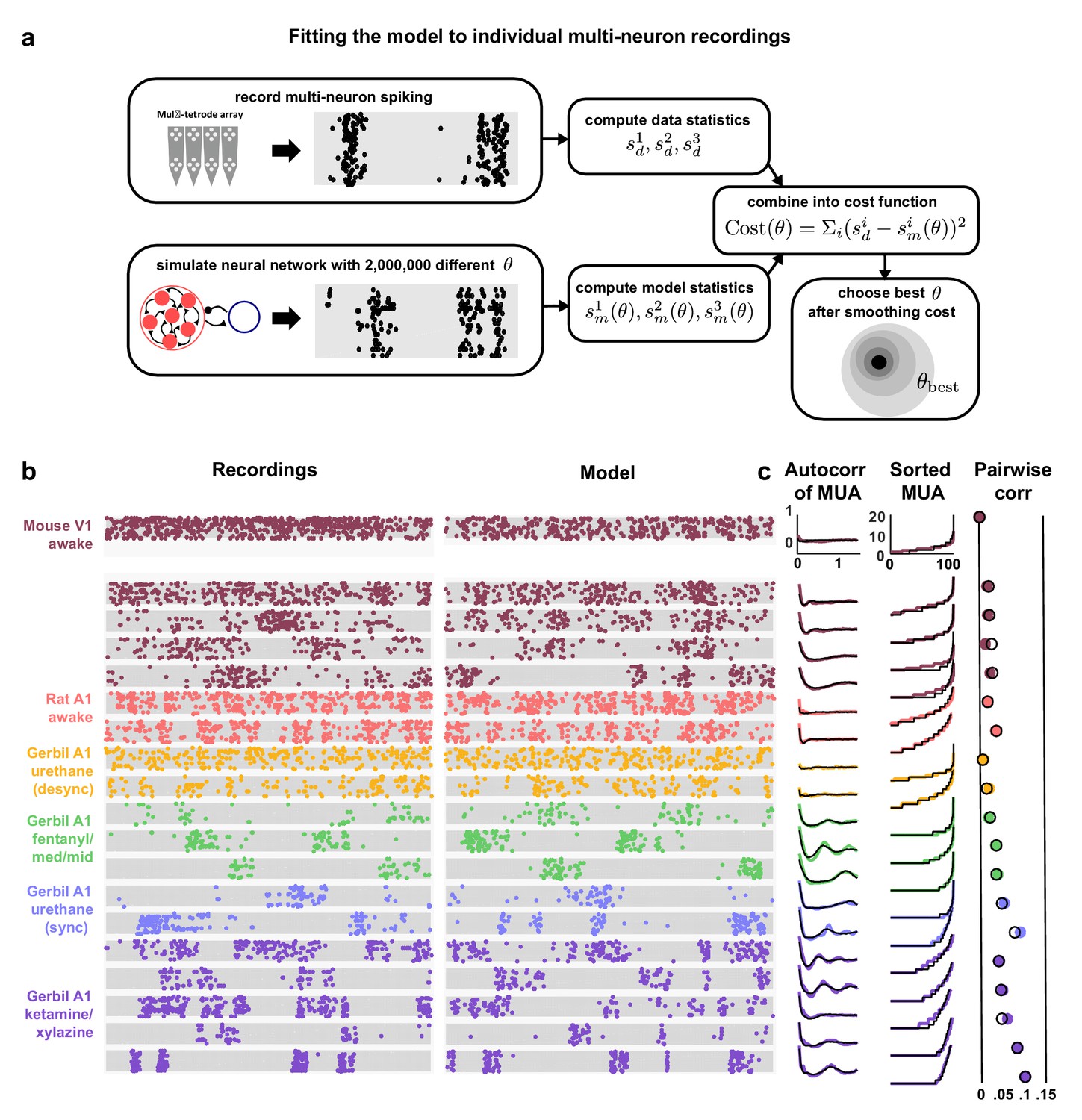
We found that increasing the strength of these inhibitory signals in the model decreased the fluctuations in electrical activity across entire areas of the brain. Further analysis of the experimental data supported the model’s predictions by showing that inhibitory neurons – which act to reduce electrical activity in other neurons – were more active when there were fewer fluctuations in activity across the brain, in both anesthetized and awake animals.
In particular, in awake mice, we observed decreases in noise correlations and increases in inhibitory activity during locomotion:
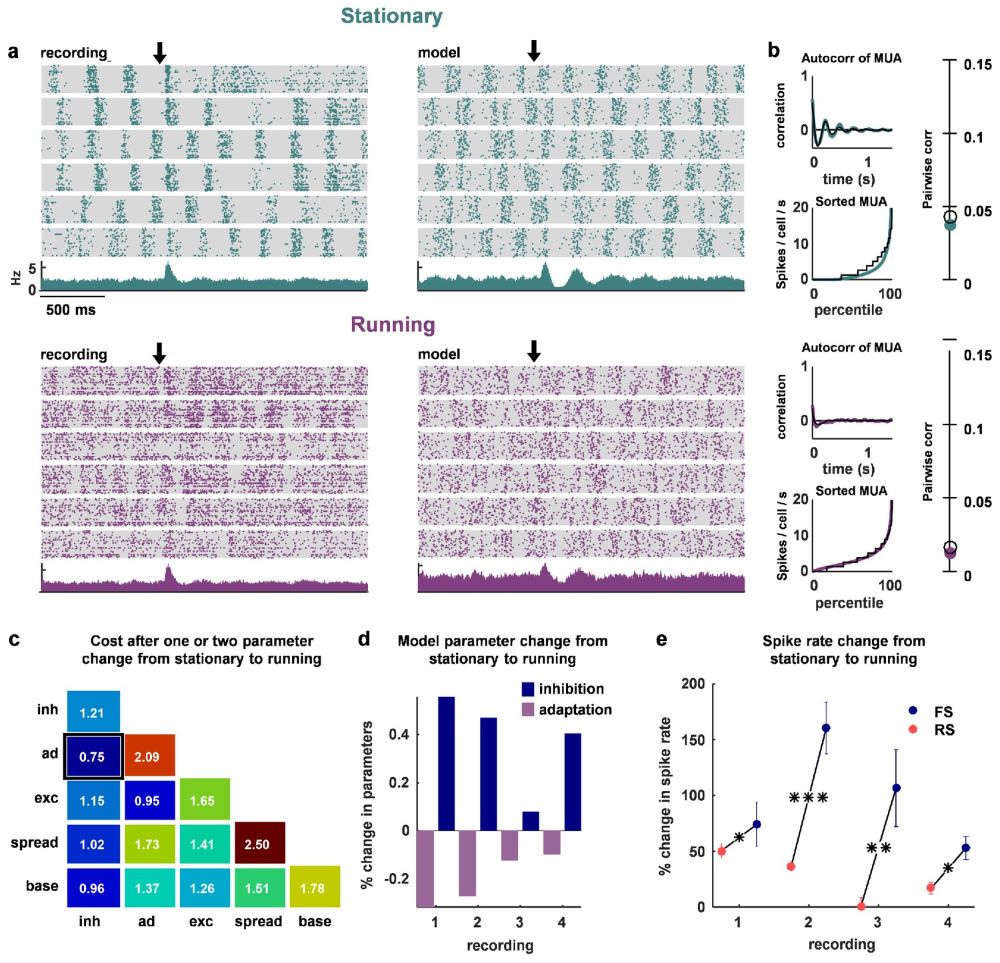
Check out the paper for more details.

Powered by Quarto. © Marius Pachitariu & Carsen Stringer lab, 2023.